Find the latest information about What Is The Charge On The Chromium Ion In Cr2o3 in this article, hopefully adding to your knowledge.
Picture this: you’re working on a chemistry experiment, and you come across a compound called Cr2O3. You know it’s an oxide of chromium, but you’re not sure what the charge on the chromium ion is. Don’t worry, I’ve been there too! Let’s dive into the world of chromium and its chemistry to unravel this mystery.
What Is The Charge On The Chromium Ion In Cr2o3
To understand the charge on the chromium ion in Cr2O3, we need to first understand the concept of oxidation states. An oxidation state tells us the hypothetical charge an atom would have if all its bonds were ionic. In Cr2O3, chromium is bonded to oxygen atoms, which are more electronegative than chromium. This means that oxygen tends to pull electrons away from chromium, giving chromium a positive oxidation state.
Chromium in Cr2O3
In Cr2O3, chromium has an oxidation state of +3. This means that each chromium ion has lost three electrons. To balance the compound, each oxygen ion has a charge of -2. Since there are two chromium ions and three oxygen ions in the formula, the overall charge of Cr2O3 is zero.
Calculating the Charge on the Chromium Ion
To calculate the charge on the chromium ion, we can use the following formula:
Charge on chromium ion = Oxidation state x Number of chromium ions
In this case, the oxidation state of chromium is +3 and there are two chromium ions, so the charge on each chromium ion is:
Charge on chromium ion = +3 x 2 = +6
Therefore, the charge on the chromium ion in Cr2O3 is +6.
Trends and Developments
The chemistry of chromium and its compounds is a vast and ever-evolving field. Recent research has focused on developing new methods for synthesizing and characterizing chromium-based materials. These materials have potential applications in a wide range of industries, including energy, electronics, and medicine.
Tips and Expert Advice
If you’re working with chromium compounds, here are a few tips to keep in mind:
- Always wear appropriate safety gear, including gloves, eye protection, and a lab coat.
- Chromium compounds can be toxic, so it’s important to handle them with care.
- If you spill a chromium compound, clean it up immediately and dispose of it properly.
- If you have any questions about working with chromium compounds, consult with a qualified professional.
By following these tips, you can help ensure your safety when working with chromium compounds.
FAQ
Q: What is the oxidation state of chromium in Cr2O3?
A: +3
Q: What is the charge on the chromium ion in Cr2O3?
A: +6
Q: Are chromium compounds toxic?
A: Yes, chromium compounds can be toxic, so it’s important to handle them with care.
Conclusion
In this article, we’ve explored the charge on the chromium ion in Cr2O3. We’ve also discussed the latest trends and developments in chromium chemistry and shared some tips for working with chromium compounds safely. I hope this information has been helpful. If you have any further questions, please don’t hesitate to reach out to me.
If you found this article interesting, please share it with your friends and colleagues. And be sure to check out my other blog posts on chromium and its compounds.
What Is The Charge On The Chromium Ion In Cr2o3
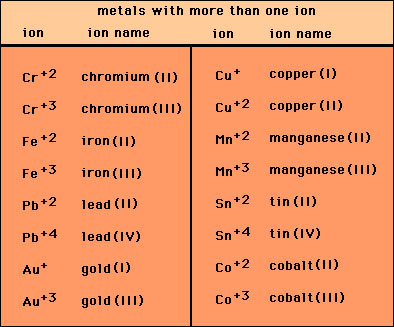
Image: apoontheweb.weebly.com
We express our gratitude for your visit to our site and for reading What Is The Charge On The Chromium Ion In Cr2o3. We hope this article is beneficial for you.